The FDA is warning the public to stop using some COVID-19 tests because they have not been authorized, cleared or approved by the FDA for distribution or use in the United States.
The FDA is concerned about the risk of false results when using these unauthorized tests:
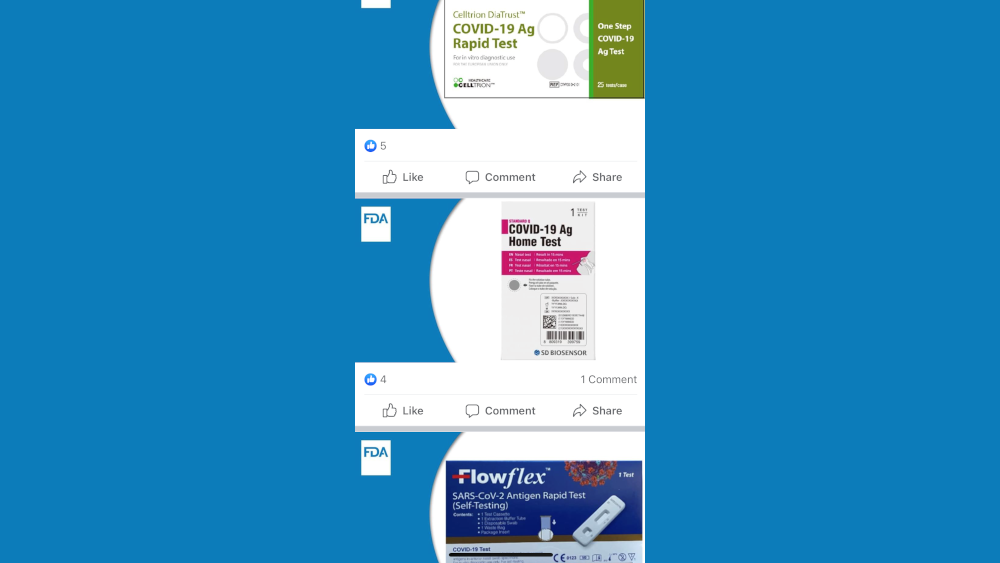
· The Celltrion DiaTrust COVID-19 Ag Rapid Test that is in green and white packaging. Find out more: https://go.usa.gov/xzjPX
· The SD Biosensor STANDARD Q COVID-19 Ag Home Test that is in white and magenta packaging.
Find out more: https://go.usa.gov/xzjPP
· The ACON Laboratories Flowflex SARS-CoV-2 Antigen Rapid Test (Self-Testing) that is in dark blue packaging.
Find out more: https://go.usa.gov/xzjPR.